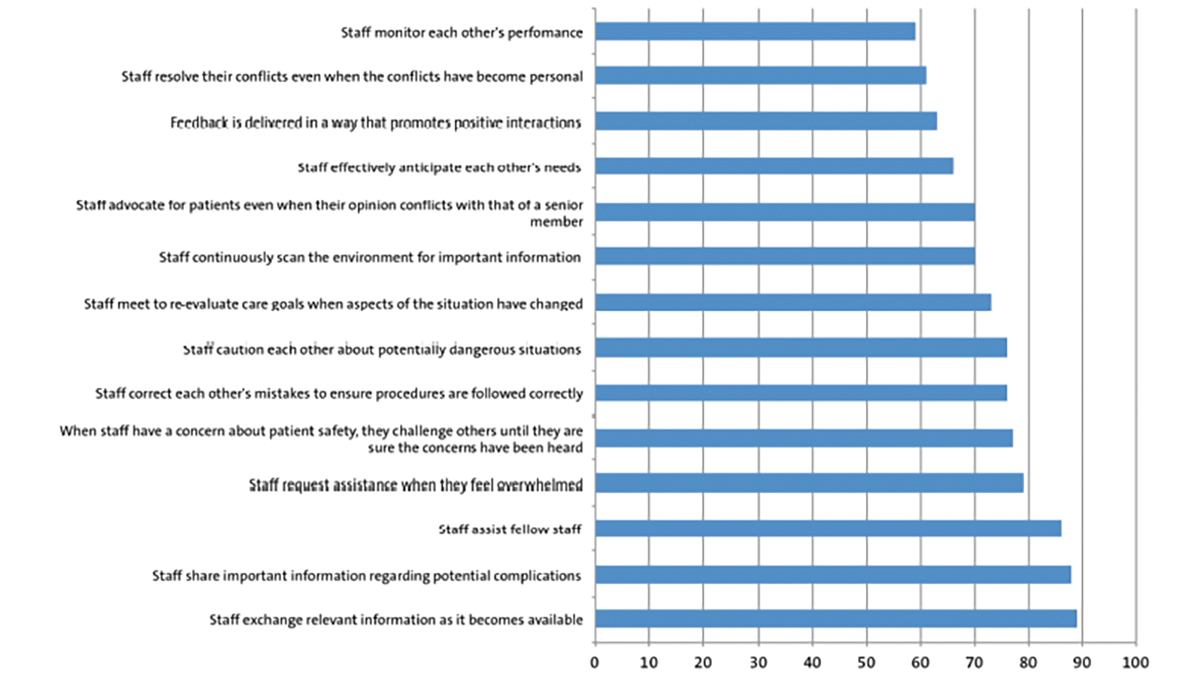
Full milk feeding from day one for preterm infants
The FEED1 trial will investigate whether full milk feeds from day 1 in infants born at 30+0 to 32+6 weeks’ gestation reduces the length of hospital stay when compared to intravenous fluids or parenteral nutrition with gradual milk feeding. Early establishment of milk feeding in preterm infants could reduce risks of infection and improve growth. Achieving fully nutritional volumes of milk feeds earlier and improving growth without infections or necrotising enterocolitis may help the infant to be ready for home sooner.
Eleanor Mitchell
Garry Meakin
Lucy Culshaw
Jon Dorling
Shalini Ojha
On behalf of the FEED1 co-applicants
(TABLE 1) and FEED1 investigators and colleagues at the Nottingham Clinical
Trials Unit.
TABLE 1 The FEED1 trial: co-applicants, investigators and colleagues. Key: NCTU = Nottingham Clinical Trials Unit.
Despite evidence to the contrary, clinicians often delay initiating milk feeds or increase feed volume slowly due to fear of necrotising enterocolitis (NEC). The recently completed Speed of Increasing milk Feeds Trial (SIFT)1 provides evidence from over 2,800 babies that faster advancement of milk feeds does not increase the risk of NEC even in the most premature infants.
Infants born at more mature gestations have a lower risk of NEC and may be able to be fully milk fed from day 1. Full milk feeds from day 1 could also avoid invasive procedures such as insertion of central venous lines and therefore reduce the risk of infections associated with such procedures. It could also reduce the number of painful procedures such as intravenous cannulations and support more involvement of families, particularly mothers, in the care of the infant from the first day of life.
Small randomised controlled trials (RCTs) have demonstrated the feasibility of giving full milk feeds from day 1.2-7 These trials found that infants randomised to full milk regained birth weight quicker, had improved growth at discharge, shorter duration of hospital stay, and fewer cases of sepsis without an increased risk of NEC. These studies are very small and were all conducted in India where the preterm population, healthcare resources, infrastructure and delivery systems as well as treatments and risk factors are different to those in the UK. There have been no studies in the UK or other similar high-resource settings investigating the strategy of feeding preterm infants ‘full milk’ feeds from day 1.
The FEED1 trial
The Fluids Exclusively Enteral from Day 1 trial (FEED1) will investigate whether full milk feeds from day 1 in infants born at 30+0 to 32+6 weeks’ gestation reduces the length of hospital stay when compared to intravenous fluids or parenteral nutrition (PN) with gradual milk feeding as per standard practice. The study will recruit participants from 40 neonatal units across the UK and include over 2,000 preterm infants (FIGURE 1).
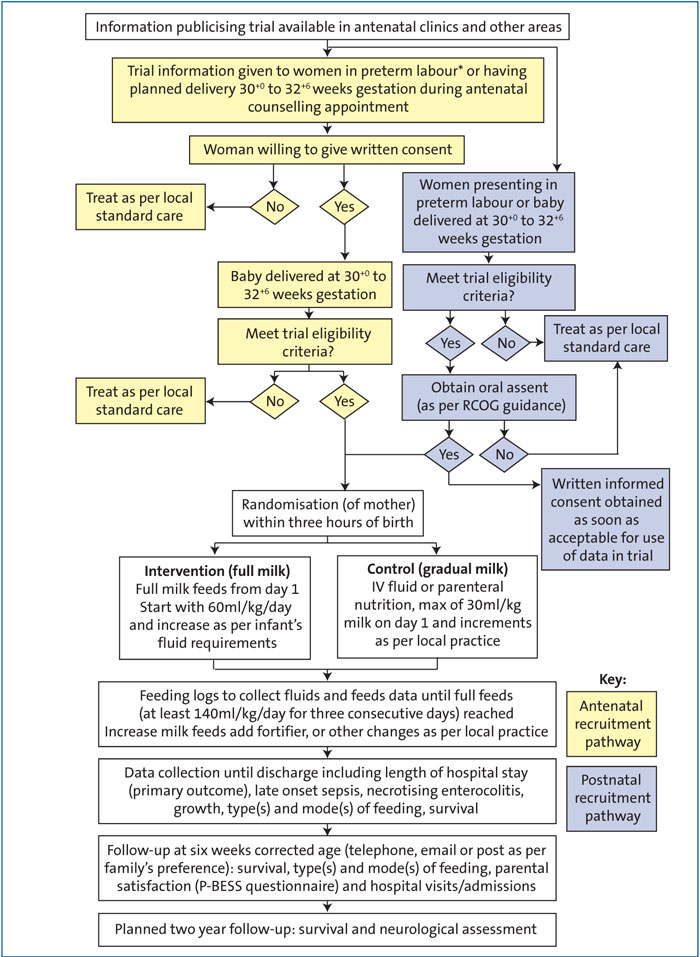
FIGURE 1 The FEED1 trial: an overview. *Women presenting in preterm labour may be consented via the antenatal or postnatal pathway. Clinician discretion will be used to determine the most appropriate method.
Outcomes
The primary outcome is length of hospital stay. Preterm infants spend long periods of time in hospital and a reduction in this time will help families come together sooner. It will also reduce the pressure of cot space in neonatal services thus reducing the cost of care for preterm infants. Early milk feeding with support to mothers to encourage breast milk expression will promote breast milk feeding and help increase breastfeeding rates at discharge.
The trial will also assess several secondary outcomes including late onset sepsis and NEC (TABLE 2). Infants will be followed up at six weeks’ corrected age.
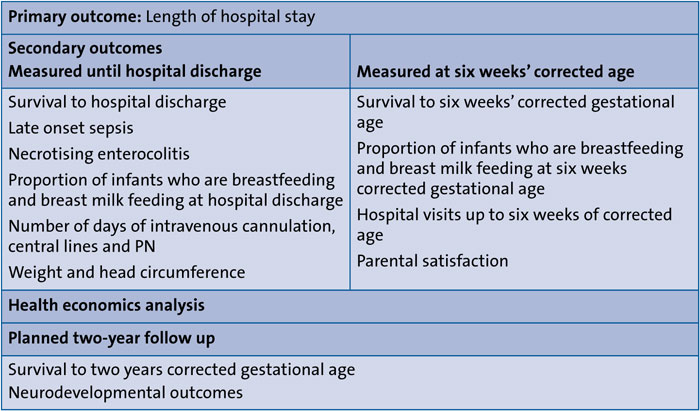
TABLE 2 Outcome measures of the FEED1 trial.
A full health economics analysis will be performed to investigate if the cost of care can be reduced by using full milk from day 1. A further follow up at two years of corrected age is planned for assessing neurodevelopmental outcomes.
Mothers of eligible infants (TABLE 3) will be randomised to ‘full milk’ (ie infants receiving all their fluid requirement as milk feeds from the day of birth) or ‘gradual milk’ (ie intravenous fluids or PN and gradual increase in milk feeds as per standard practice). The mother will be randomised so that siblings from multiple births such as twins and triplets will receive the same intervention and so that antenatal discussions and consent can be obtained in many but not all cases.

TABLE 3 Inclusion criteria for the FEED1 trial.
Recruitment and randomisation of mothers: the need to randomise and start intervention within three hours of birth
Infants need to be entered into the trial within three hours of birth (FIGURE 1). Mothers expecting to deliver at 30+0 to 32+6 weeks’ gestation will be approached, where possible, in the antenatal period or immediately after birth. The immediate hours after birth are often a very stressful time for mothers and families. It may not be appropriate to burden families with detailed information about the trial and ask for written consent at such worrying times. Previous studies have looked into the consent process immediately after birth and based on their results the Royal College of Obstetrics and Gynaecology (RCOG) has recommended that a process involving oral assent followed by deferred written consent is more suitable for perinatal trials that require participants
to be entered in a time critical manner. Following this advice, due to the short time available to randomise mothers and enter infants into the trial, if antenatal consent has not previously been provided mothers will be initially asked for oral assent. If the mother agrees to participate, the oral assent will be documented, randomisation performed and the infant(s) entered into the trial. Following this, written informed consent will be taken as soon as possible to allow the participants’ data to be entered into the trial.
Full milk from day 1 vs gradual milk
Infants in the full milk arm will receive all their fluid requirements as milk. Milk feeds will be started at 60mL/kg/day. Frequency of feeding will be as per local practice, such as two-hourly, hourly or continuous feeding. The choice of milk will depend on the mother’s preference and local practice. Mother’s own breast milk will always be the first choice and all mothers will be supported to express breast milk for their infants. In the first few days, the volume of the mother’s milk may not be sufficient to fully milk feed her infants. The volume will be supplemented with human donor breast milk or preterm infant formula milk as per mother’s choice and local practice. Any extra milk, whether human donor breast milk or infant formula will only replace the intravenous fluids or PN that the infant would have received in normal clinical practice. Mother’s milk will always be given to the infant and the aim is to provide the total required volume as mother’s own milk as soon as possible. Milk feed volumes will be increased gradually at a rate such that all the infant’s fluid requirements are met with milk.
In the gradual milk group, infants will receive intravenous fluids, PN, and smaller volumes of milk feeds increased as per local practice.
Daily feeding logs will be used to collect data on feeding and fluids until the infant reaches full feeds (defined as at least 140mL/kg/day of milk feeds for three consecutive days). Data on other outcomes, such as late onset sepsis and NEC will be collected until discharge.
Progress of the trial and contact information
The trial has been funded by the National Institute of Health Science Health Technology Assessment Programme (project: 17/94/31). It has received ethical approval from the East Midlands-Derby Research Ethics Committee (ref: 19/EM/0258). Sites have been selected and training has begun. The first set of sites began recruiting in October 2019. Full trial protocol, study information leaflets, and further information can be requested by contacting feed1@nottingham.ac.uk or via Twitter @FEED1Trial.
Discussion
The full milk feeds from day 1 intervention is aimed at ‘normalising’ the care of preterm infants who are at a lesser risk of complications of large volume milk feeds such as NEC and may be able to safely feed fully from the first day after birth. It will encourage mothers and families to be involved in the infant’s care from the first day and support mothers to express breast milk.
Preterm infants born at 30+0 to 32+6 weeks’ gestation are unable to breastfeed directly due to their immature sucking and swallowing. In current practice, their fluid requirements are met by intravenous fluids or PN. The infants have to undergo painful procedures such as intravenous cannulation and often more invasive procedures, for example placement of central venous lines. These increase the risk of serious infections such as late onset sepsis.
According to current practice and accepted medical knowledge, a preterm infant requires 60mL/kg/day to maintain haemodynamic stability. Unlike term infants who can thrive on small volumes of colostrum for the first few days, preterm infants will become unwell if given such small volumes. Therefore, even if the preterm infant receives some volumes of mother’s milk in the first few days, the infant’s fluid requirements have to be met with extra fluids. In current practice, this is met with intravenous fluids or PN, which come with risks of painful procedures, invasive infections, and electrolyte imbalances. The FEED1 trial aims to replace PN or other intravenous fluids with milk feeds. As mothers produce small volumes of colostrum in the first few days, it is very likely that infants randomised to the full milk arm will require some supplementation of the mother’s expressed breast milk with human donor milk or infant formula on the first few days of life. Mother’s own breast milk will always be given first and the trial protocol aims to fully milk feed with mother’s milk as soon as adequate volumes are available.
The FEED1 trial and the team members fully support the Unicef Baby Friendly Initiative and aim to encourage early mother’s milk feeding in all participants. The supplementary milk will not be given in place of mother’s milk and will only replace the need for intravenous cannulation, placement of central lines, and infusion of glucose and electrolyte solutions or parenteral fluid. The infants will receive colostrum and all the volumes of maternal breast milk with an aim to give the full feeds with mother’s milk as soon as possible. Other breast milk feeding practices such as the use of oropharyngeal colostrum will be continued as per routine local practice and will be encouraged in both arms of the trial. All participating mothers will receive full support for expressing breast milk and establishing full breastfeeding as per local policy. Encouraging full milk feeding from the first day and supporting mothers to breastfeed may improve the rates of breastfeeding and breast milk feeding. Data on breastfeeding will be collected at discharge and at six weeks of corrected gestational age to investigate this.
Having a baby in the neonatal unit is a very stressful time for families. Further stress is added when parents see their fragile infants undergo painful procedures and being attached to numerous drips and lines. Milk feeding is less invasive and more physiological. It will reduce the ‘over-medicalisation’ of preterm care. A reduction in the length of hospital stay will reduce the time of separation and reduce the burden on families.
The FEED1 trial is expected to provide evidence that will help to improve feeding practices and care of preterm infants and will support families to be more involved in neonatal care.
The full trial protocol, study information leaflets, and further information can be requested by contacting feed1@nottingham.ac.uk or via Twitter @FEED1Trial.
Or read this article in our
Tablet/iPad edition
- FEED1 is the first large RCT to investigate whether infants born at 30+0 to 32+6weeks’ gestation will be able to milk feed fully from day 1.
- Full milk feeds from day 1 could ‘de-medicalise’ the care of preterm infants, help to avoid invasive procedures, reduce the length of their stay in neonatal units, encourage mothers to express milk, and support early breast milk feeding.
Also published in Infant:
preterm infant studies show a specific HMO, called
disialyllacto-N-tetraose (DSLNT), is present in maternal milk in lower amounts in infants who go on to develop NEC. This article reviews the role of HMOs in NEC development and the clinical data in preterm infants, and considers the possible next steps for supplementation in preterm infants.