SIGNEC Sixth International Conference on Necrotising Enterocolitis
The sixth SIGNEC (special interest group in necrotising enterocolitis) international conference was held on 29-30 October 2018 at Chelsea Football Club, London. This year day one focused on NEC definitions and datasets while day two considered the family experience, long-term concerns for babies post-NEC and recent advances in diagnosis and quality improvement.
This supplement is based on a two-day conference that was supported by an educational grant from Nutricia Early Life Nutrition.
MBBS, MD, FRCPCH, FRSA, Fellowship in Neonatal Intensive Care
Consultant in Neonatal Medicine, Poole Hospital NHS Foundation Trust, and Professor of Perinatal Health, Bournemouth University
mineshkhashu@gmail.com

Some of the speakers and chairpersons at SIGNEC 2018. From left: Minesh Khashu, Ravi Mangal Patel, Misty Good, Marie Spruce, Caron Parsons, Nigel Hall, Gopi Menon, Andrew Ewer, Kate Costeloe, Christopher Stewart and Joanne Ferguson.
NEC: the defining moment
SIGNEC founder Professor Minesh Khashu explained to the audience why one of the main obstacles to improving outcomes is the lack of universal definitions for NEC and NEC subgroups. This has led to ‘contaminated datasets’ that restrict our ability to extract useful information and gain insight. It is crucial for the global neonatal community to come to a consensus regarding NEC definitions.
Professor Khashu shared his thoughts on artificial intelligence technologies in health care and the potential use of predictive monitoring and machine learning in diagnosis and treatment for NEC. This involves analysing physiological data to identify infants at high risk of NEC and/or potentially life-threatening scenarios. The early detection of abnormal physiological patterns has the potential to predict morbidity or mortality so that appropriate treatment can be given at an early stage and thus improve outcomes.
What is wrong with NEC data? A subset approach to NEC
In his talk, Professor Phillip Gordon reiterated why we must redefine NEC. NEC data consist of many entities, some of which are not NEC. These include spontaneous intestinal perforations (SIP), food protein-induced enterocolitis syndrome, and NEC that occurs from pre-existing conditions associated with term infancy or congenital anomalies. Most of these are fundamentally different versions of disease when compared to preterm NEC. Professor Gordon stressed that future definitions of NEC must seek to exclude and tabulate these confounding factors.
One fundamental concern is that preterm NEC consists of several subgroups, although these are increasingly becoming better defined. Professor Gordon summarised the subgroup entities and gave references in the literature for further review. He discussed the concepts regarding NEC subgrouping and how we might move forward with this. He affirmed Professor Khashu’s assertion that the critical next step is to correctly define NEC and clean-up our datasets.
Professor Gordon went on to further discuss artificial intelligence-based medical diagnostics and how these might, or might not, work for NEC.
Current NEC definitions and considerations in redefining NEC
In 1978, Dr Martin Bell and colleagues proposed the first clinical staging for NEC. Dr Ravi Mangal Patel described how, 40 years later, this remains the most commonly applied criteria for the diagnosis of NEC. Potential limitations of Bell staging include: contamination from SIP; high incidence of stage I; uncertainty of the presence of pneumatosis; lack of accounting for baseline risk; and the case-definition not being explicit. Since the initial report of Bell staging, multiple approaches to diagnose or stage NEC have been proposed.1-8
During this session, the following seven considerations were proposed for redefining NEC:
- Address possible contamination by SIP.
- Avoid inclusion of Bell stage I (or equivalent cases).
- Incorporate risk-stratification into definitions (eg gestational age).
- Assess predictive ability of measures (eg abdominal tenderness) to guide inclusion.
- Compare performance of case definitions in classifying important outcomes among infants with NEC.
- Describe how uncertainty is addressed (eg findings of questionable or possible pneumatosis).
- Incorporate tools to estimate pre-test probability of NEC before diagnostic testing.
Throughout day one the audience was invited to offer opinions on adopting a new definition for NEC that incorporates subsets. The results will be collated and published in due course.
Our NEC journey
Marie Spruce, a paediatric critical care nurse and team member of NEC UK (a charity dedicated to supporting families affected by NEC, research innovation and family-centred care) presented her journey with her son Freddie who developed severe NEC resulting in short bowel syndrome, intestinal failure and home parenteral nutrition (PN). Freddie spent eight months in hospital; he had multiple surgeries before being discharged home on PN. He is now two-and-a-half years old with developmental delay and his future is uncertain. Marie discussed how professionals could better support families with long-term admissions and looked at the difficulties she faced while in hospital, in particular the use of donor milk, quality of life discussions, early discharge planning, involving families in care and acknowledging parental concerns. She gave an insight into life with a child on home PN to highlight awareness of the difficulties faced and rounded off by discussing her involvement with NEC UK, the charity’s achievements and ongoing plans.
Intestinal rehabilitation and intestinal transplantation
Loss of small intestine in NEC can lead to short bowel syndrome and chronic intestinal failure. Intestinal rehabilitation aims to wean a child off PN, which can take years to achieve and sometimes requires intestinal transplantation. Dr Girish Gupte described
the roles of intestinal rehabilitation and transplantation in the treatment of NEC and their implementation in practice. He presented data on national and international outcomes in this area. He highlighted the importance of early referral to the transplant team; children with intestinal failure benefit more from early intervention and management.
Dr Gupte enlightened the audience with a description of local regional practice in Birmingham and the Midlands and once again encouraged clinicians to have earlier discussions with the transplant team. Earlier referral and innovative surgical strategies have resulted in improved long-term survival of children referred for intestinal transplantation.
Improving the long-term cognitive function of babies with NEC: from animal models to potential therapies
Dr David Hackam reminded the audience that one of the most important long-term complications observed in children who survive NEC early in life is the development of severe neurological impairment. Remarkably, the pathways leading to NEC-associated neurological impairment remain unknown, thus limiting the development of prevention strategies. The Hackam laboratory has shown in prior studies that NEC development requires the expression of the lipopolysaccharide receptor toll-like receptor 4 (TLR4) on the intestinal epithelium, activation of which by bacteria in the newborn gut leads to mucosal inflammation.
In seeking to understand the development of NEC-associated neurological impairment, Dr Hackam’s team has shown that TLR4 signalling in the intestine leads to a dramatic release of the ‘danger molecule’ HMGB1, leading to the activation of microglial cells in the brain, reduced myelination and cognitive impairment. They have also identified a gut-brain signalling axis in the premature host in which intestinal TLR4 signalling in NEC leads to intestinal epithelial HMGB1 release, leading to activation of microglia and neurological dysfunction. Strikingly, Dr Hackam further demonstrated that an orally administered dendrimer-based nano-therapeutic approach to target activated microglia can prevent NEC-associated neurological dysfunction in neonatal mice. These findings reveal the molecular pathways leading to the development of NEC-associated brain injury, and also provide a justification for early removal of diseased intestine in NEC in order to prevent ongoing brain injury. Dr Hackam provided a rationale for the further development of targeted therapies that protect the devel-oping brain, which may offer new hope for those who develop NEC in early childhood.
Quality improvement to reduce NEC
Dr Gopi Menon described how NEC is one of the most important unsolved neonatal morbidities. There is considerable variation
in incidence and associated mortality. Quality improvement is generally not amenable to off-the-shelf solutions and needs to be tailored to suit local circumstances; Dr Menon illustrated this with examples of what has been done in his unit in Edinburgh.
Quality improvement is best progressed by:
- benchmarking with similar units
- identifying potentially better practices from the literature and from colleagues
- developing a driver diagram identifying the systems and processes involved and forces influencing these
- forming a multi-professional quality improvement group including parent representation
- giving attention to focusing effort where it is most needed
- working on wide staff engagement
- understanding that it is best done as an iterative process using small tests of change, but that it is never complete.
The conditions are right in the UK for national collaboration on quality improvement and the British Association of Perinatal Medicine (BAPM) is developing resources to help this.
Novel treatment approaches to NEC: hope on the horizon
Dr Misty Good’s laboratory focuses on the signalling pathways involved in the mucosal immune response during NEC and how these responses can be modified or prevented through dietary modifications or targeted intestinal epithelial therapies. Utilising a humanised neonatal mouse model of intestinal injury, Dr Good’s team has discovered an immunomodulatory approach to preventing experimental NEC and is determining the mechanisms involved in this protection. Dr Good is currently working with the USA Food and Drug Administration agency on a future clinical trial with this therapeutic strategy for NEC in premature infants. Furthermore, she reviewed several key research techniques performed in her laboratory including human and mouse intestinal epithelial and stem cell culture, as well as the development of the first ‘NEC-on-a-chip’ microfluidic model system. Taken together, these novel approaches to new experimental model systems of NEC along with a promising drug discovery, offer families and clinicians hope on the horizon for the prevention and treatment of this devastating disease.
NEC Awareness Day: 17 May
Joanne Ferguson described how SIGNEC worked with partners at the NEC Society, the PGG Institute in Brazil and NEC UK to make 17 May 2018 the first day ever dedicated to this devastating disease. Parents and professionals around the world joined together to raise awareness of NEC and, as it was agreed that there should be more emphasis on clinicians and researchers in future, delegates were asked to contribute ideas at a unit and global level to mark the occasion in 2019.
The role of ultrasound in the diagnosis of NEC
The abdominal X-ray is the current standard imaging technique for diagnosis and follow-up of NEC, however, the early signs of NEC are neither sensitive nor specific. Dr Caron Parsons explained why ultrasound should be considered as an adjunct to the abdominal
X-ray and has several advantages: it allows real-time direct visualisation; it has the potential to appropriately stage NEC; it involves no radiation; it is a bedside study, and it provides prog-nostic information. Real-time direct visualisation of the neonatal bowel allows evaluation of bowel wall thickening/thinning, echogenicity, perfusion and peristalsis. Identifying free fluid and focal collections has a significant impact on diagnostic certainty. Ultrasound is more sensitive for the detection of pneumatosis, portal venous gas, free gas, fluid and the quality of fluid.
Distinguishing NEC from other acquired neonatal intestinal diseases, such as SIP and septic ileus, is often difficult as there can be an overlap of signs. However, particular attention should aim to identify decreased Doppler signal in mesenteric vessels and the bowel wall, echogenic free fluid and bowel wall thinning. Consistent ultrasound settings and technique should be maintained to allow recognition of both the normal and pathological neonatal bowel.
Dr Parsons emphasised that ultrasound is widely available, easy to perform following training and should be encouraged in the context of a multidisciplinary approach.
Infant microbiome in health and disease: current understanding and future direction
Dr Christopher Stewart provided an overview of how the gut microbiome develops in term and preterm infants. In the first part of his talk, Dr Stewart talked about his recent work on the TEDDY cohort, a study of over 12,000 stool samples from nearly 1,000 infants that underwent microbiome sequencing. The results showed that diet (receipt of breast milk or not) is the major factor associated with the bacterial community in infants in early life. Specifically, breastfeeding increased the relative abundance of Bifidobacterium in the gut. In the second part of his talk, Dr Stewart summarised his published and ongoing research on the preterm gut microbiome in NEC and sepsis. In comparison to healthy controls, preterm infants who go on to develop NEC or sepsis have reduced bacterial diversity, lower relative abundance of Bifidobacterium, and a less stable development of the mircobiome over the initial weeks of life. Dr Stewart’s laboratory is now building on this work to investigate the underlying disease mechanisms and determine if microbial therapy (eg probiotics) represent a tangible option for reducing the incidence of NEC and sepsis in preterm infants.
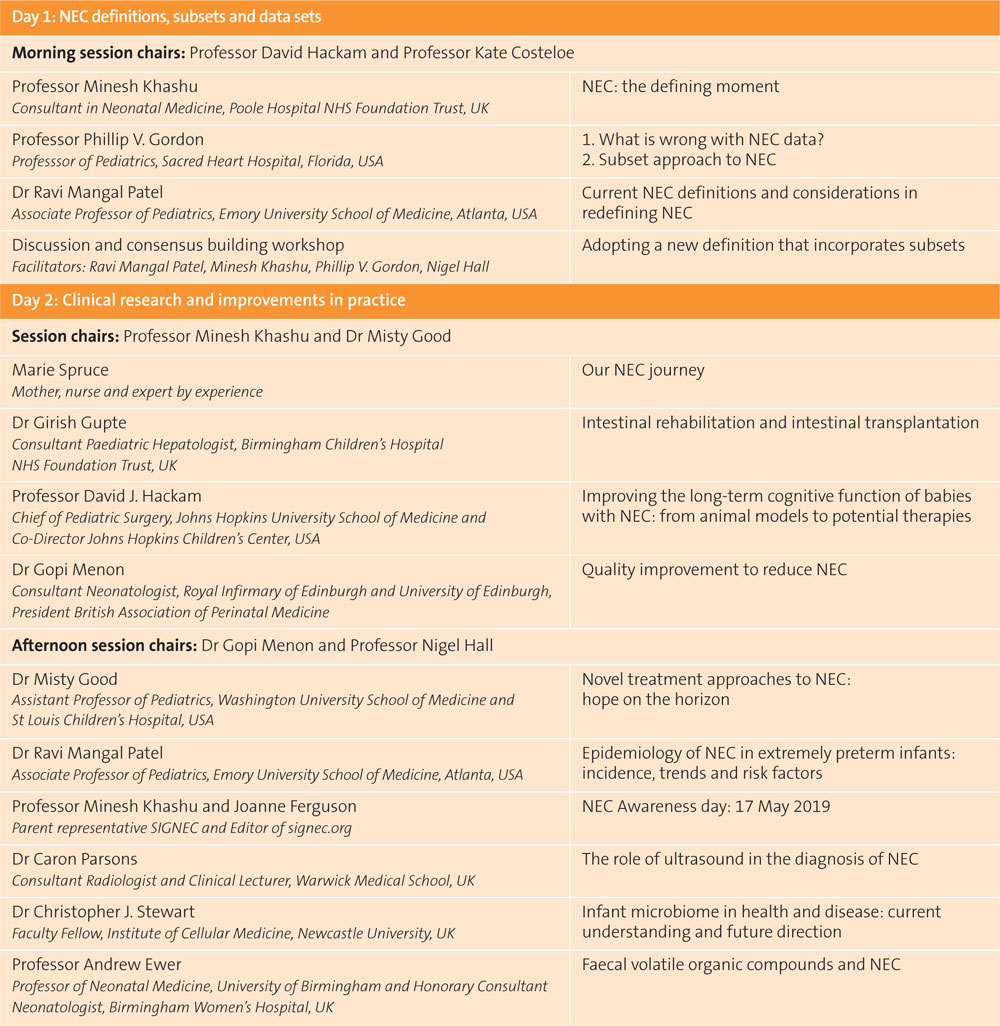
TABLE 1 Programme of speakers.
Some presentations from the conference appear on the website at signec.org with kind permission of the speakers. For updates on the seventh SIGNEC conference contact mineshkhashu@gmail.com or visit the website. Neonatal teams are encouraged to use the website for education and signposting for parents.
Or read this article in our
Tablet/iPad edition